#azeotrope
Azeotrope
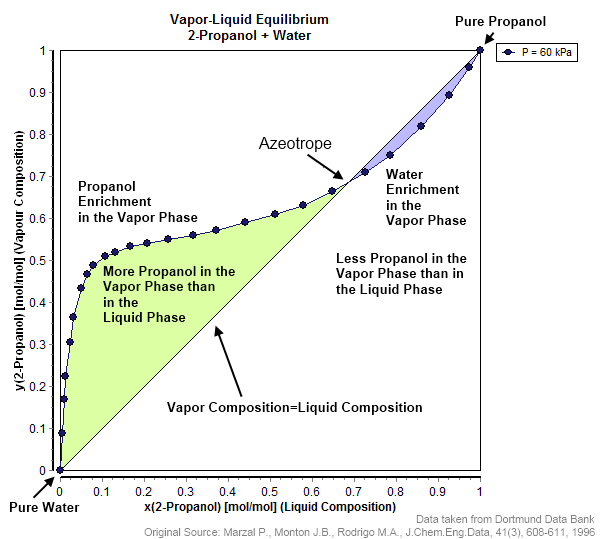
Mixture of two or more liquids whose proportions do not change when the mixture is distilled
An azeotrope or a constant heating point mixture is a mixture of two or more components in fluidic states whose proportions cannot be altered or changed by simple distillation. This happens because when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Azeotropic mixture behavior is important for fluid separation processes.
Tue 4th
Provided by Wikipedia
This keyword could refer to multiple things. Here are some suggestions:
0 searches
This keyword has never been searched before
This keyword has never been searched for with any other keyword.