#hydration_number
Hydration number
Measure of solvency/solution
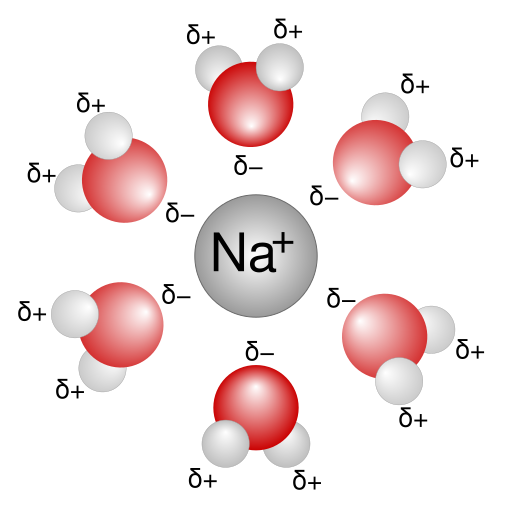
The hydration number of a compound is defined as the number of molecules of water bonded to a central ion, often a metal cation. The hydration number is related to the broader concept of solvation number, the number of solvent molecules bonded to a central atom. The hydration number varies with the atom or ion of interest.
Sat 8th
Provided by Wikipedia
This keyword could refer to multiple things. Here are some suggestions:
0 searches
This keyword has never been searched before
This keyword has never been searched for with any other keyword.