#itaconic_acid
Itaconic acid
Chemical compound
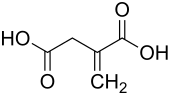
Itaconic acid (also termed methylidenesuccinic acid and 2-methylidenebutanedioic acid) is a fatty acid containing five carbons (carbon notated as C), two of which are in carboxyl groups (notated as -CO2H) and two others which are double bonded together (i.e., C=C). (itaconic acid's chemical formula is C5H6O4, see adjacent figure and dicarboxylic acids). At the strongly acidic pH levels below 2, itaconic acid is electrically neutral because both of its carboxy residues are bound to hydrogen (notated as H); at the basic pH levels above 7, it is double negatively charged because both of its carboxy residues are not bound to H, i.e., CO2 (its chemical formula is C5H4O42-); and at acidic pH's between 2 and 7, it exists as a mixture with none, one, or both of its carboxy residues bound to hydrogen. In the cells and most fluids of living animals, which generally have pH levels above 7, itaconic acid exists almost exclusively in its double negatively charged form; this form of itaconic acid is termed itaconate. Itaconic acid and itaconate exist as cis and trans isomers (see cis–trans isomerism). Cis-itaconic acid and cis-itaconate isomers have two H's bound to one carbon and two residues (noted as R) bound to the other carbon in the double bound (i.e., H2C=CR2) whereas trans-itaconic acid and trans-itaconate have one H and one R residue bound to each carbon of the double bound. The adjacent figure shows the cis form of itaconic acid. Cis-aconitic acid spontaneously converts to its thermodynamically more stable (see chemical stability) isomer, trans-aconitic acid, at pH levels below 7. The medical literature commonly uses the terms itaconic acid and itaconate without identifying them as their cis isomers. This practice is used here, i.e., itaconic acid and itaconate refer to their cis isomers while the trans isomer of itaconate (which has been detected in fungi but not animals) is here termed trans-itaconate (trans-itaconic acid is not further mentioned here).
Mon 19th
Provided by Wikipedia
This keyword could refer to multiple things. Here are some suggestions: