#ocean_acidification
Ocean acidification
Decrease of pH levels in the ocean
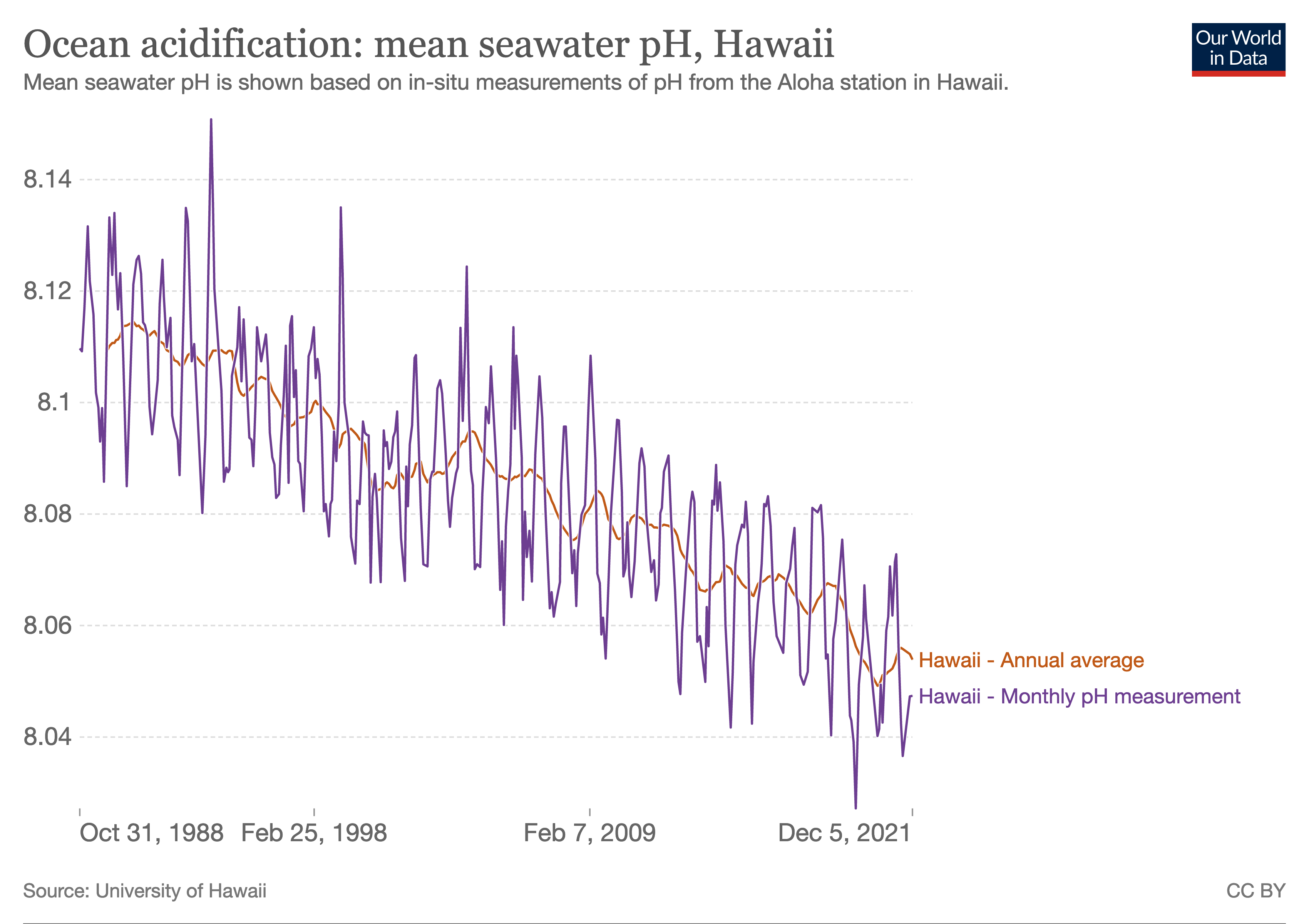
Ocean acidification is the ongoing decrease in the pH of the Earth's ocean. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05. Carbon dioxide emissions from human activities are the primary cause of ocean acidification, with atmospheric carbon dioxide levels exceeding 410 ppm. CO2 from the atmosphere is absorbed by the oceans. This chemical reaction produces carbonic acid which dissociates into a bicarbonate ion and a hydrogen ion. The presence of free hydrogen ions lowers the pH of the ocean, increasing acidity. Marine calcifying organisms, such as mollusks and corals, are especially vulnerable because they rely on calcium carbonate to build shells and skeletons.
Thu 5th
Provided by Wikipedia
This keyword could refer to multiple things. Here are some suggestions: