#salt_bridge
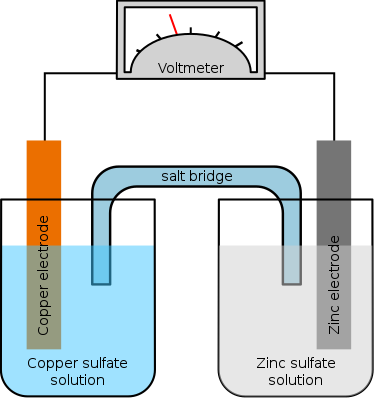
Salt bridge
Laboratory device used for electrochemistry
In electrochemistry, a salt bridge or ion bridge is an essential laboratory device discovered over 100 years ago. It contains an electrolyte solution, typically an inert solution, used to connect the oxidation and reduction half-cells of a galvanic cell, a type of electrochemical cell. In short, it functions as a link connecting the anode and cathode half-cells within an electrochemical cell. It also maintains electrical neutrality within the internal circuit and stabilizes the junction potential between the solutions in the half-cells. Additionally, it serves to minimize cross-contamination between the two half cells and helps concentrate our focus on unfolding the function of working electrodes of the half-cells.
Wed 18th
Provided by Wikipedia
This keyword could refer to multiple things. Here are some suggestions: