#thermodynamic_versus_kinetic_reaction_control
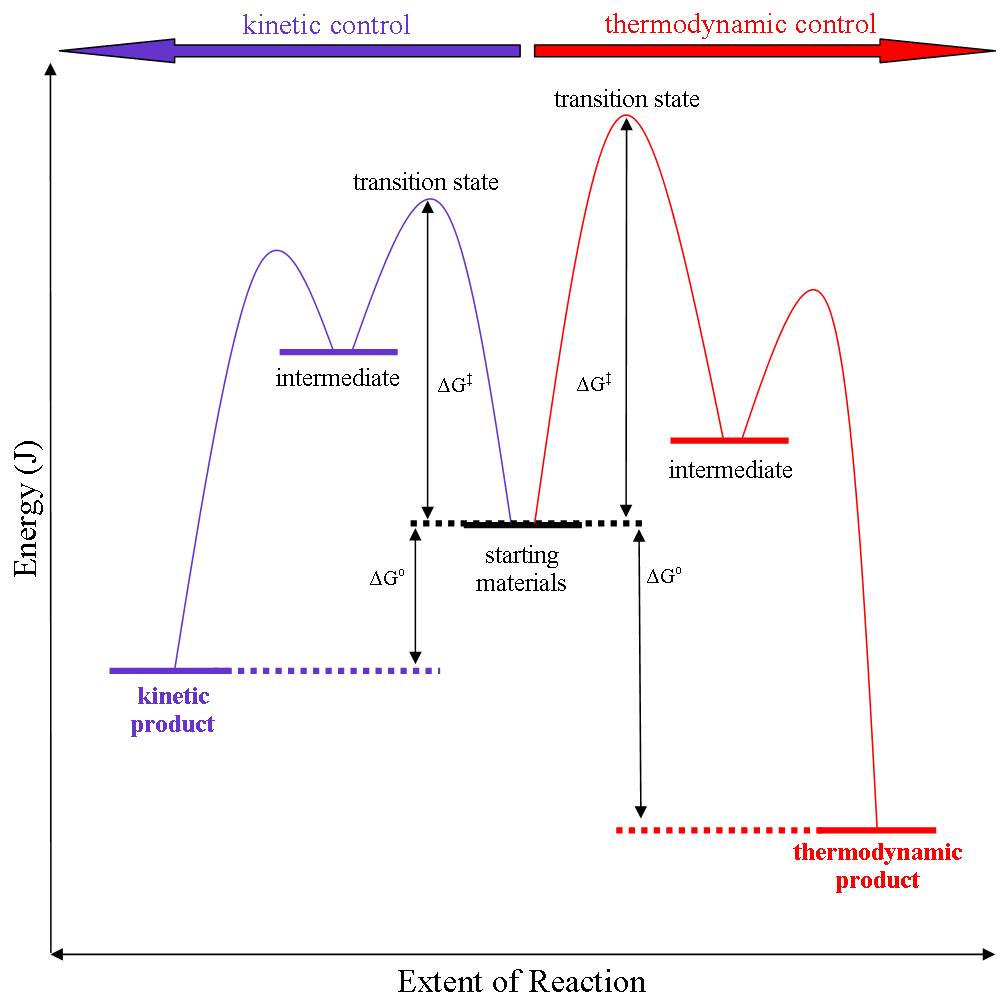
Thermodynamic versus kinetic reaction control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity or stereoselectivity. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favoured under kinetic control and B is the thermodynamic product and is favoured under thermodynamic control.
Thu 9th
Provided by Wikipedia
This keyword could refer to multiple things. Here are some suggestions: